Medical Devices
Healthcare providers and IT vendors in the region have weighed in with their predictions for healthcare technology in the new year.
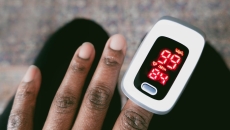
The U.S. Food and Drug Administration has published updated draft recommendations to help improve the performance of pulse oximeters across skin tones.
After resolving how it will review updates to previously approved artificial intelligence-enabled medical devices, the agency will release its full draft proposal for market submissions.
Also, AIIMS Delhi is setting up a hub for healthcare AI development with GE HealthCare.
"The usage of AI and automatic vulnerability scanning performed by the attackers allows them to find an exposed IoT device and conduct an attack on it much quicker than they used to be able to," says one security researcher in a new report.
It will feature a 24/7 command centre, integrating the ICUs of small and medium hospitals.
The bipartisan continuing resolution announced on Monday offers "big wins" for virtual care, and the American Telemedicine Association and other healthcare groups are pleased.
The safety nonprofit says artificial intelligence models that aren't properly evaluated and deployed are a big concern for 2025. Others include home health technology and hospital infusion system vulnerabilities.
Also, the South Korean government will pilot an electronic system to maintain the patient medical records at shuttered health facilities.
The agency said it will review developers' modified Predetermined Change Control Plans for artificial intelligence and machine learning submissions that have already been approved without triggering the need for new marketing submissions.