Medical Devices
"The Department of Justice is committed to prosecuting people who abuse our healthcare system and exploit telemedicine technologies in fraud and bribery schemes," says assistant AG.
HIMSS22 Europe
Elad Benjamin, general manager at Clinical Data Services at Philips, sheds light on findings from the latest Philips Future Health Index report, including the effects of staff shortages on healthcare.
The transaction, meant to bolster Evolent's value-based specialty care offerings, could eventually be worth an extra $87 million, depending on future performance milestones.
Becton, Dickinson and Company will gain access to de-identified real-world evidence from Mayo Clinic Platform_Discover, using AI for insights into medical device safety and security in order to streamline its regulatory submissions.
HIMSS22
Sumit Nagpal, CEO and founder of Cherish Health, explains the use of ambient sensing for remote monitoring of aging patients without wearables or cameras.
A new report from the Cloud Security Alliance explains that it's essential to apply a risk rating, using predefined criteria, to all subscribers.
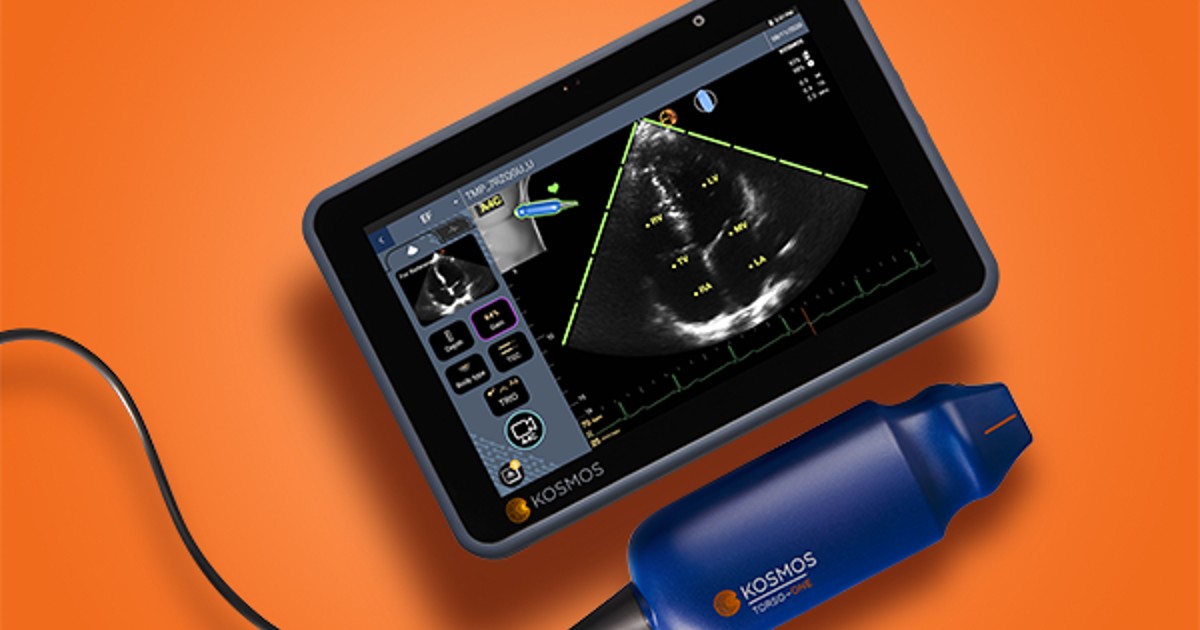
Dr. Mark Favot says learning to use point-of-care ultrasound devices can be "very humbling" for physicians – but artificial intelligence can make it easier for them to acquire new skills.
The agency is seeking stakeholder feedback on the guidance, which seeks to clearly outline its recommendations for premarket submission content when it comes to cybersecurity concerns.
UCLA Biodesign executive director Dr. Jennifer McCaney shares insights on a recent research project with BCG examining the global regulatory landscape, and discusses what excites her about the industry's future.
The bipartisan bill would put in place baseline cybersecurity requirements for device manufacturers applying for FDA approval and require plans to monitor and address post-market vulnerabilities.